A Carboxyl Group Contains Which of the Following Atoms
The general formula of amino acids is given as. The carboxyl group is seen in many organic molecules known as carboxylic acids which have a variety of functionsThe carboxyl group consists of a carbon bonded to both an oxygen and a hydroxyl group.

Review The Functional Groups In Organic Chemistry Functional Group Organic Chemistry Chemistry
The carboxyl is commonly written as -C OOH or -COOH.
. Organic compounds that contain carboxyl groups are called carboxylic acids. The carboxyl is an organic functional group consisting of a carbon atom double-bonded to an oxygen atom and singly bonded to a hydroxyl group. Since hydrocarbons by definition contain only carbon and hydrogen a.
The simplest carboxylic acid is the alkanoic acids. The carboxyl group is a functional group that contains a carbonoxygen double bond and an OH group also attached to the same carbon atom but it has characteristic properties of its own. A carboxyl group COOH is a functional group consisting of a carbonyl group CO with a hydroxyl group O-H attached to the same carbon atom.
The carboxyl group occurs on the end or side of a molecule. Carboxyl functional group the carboxyl group COOH contains two O atoms that pull electrons away from the H atom so this group loses a proton and is acidic. 1 Carbon 2 oxygen 1 hydrogen.
It also has a single bond to a hydroxyl group. What atoms make up the Carboxyl Groups. D the chemical properties of their amino and carboxyl groups.
It has one two or all three of the hydrogen atoms of NH 3 replaced by an alkyl or. 31 Peptide bonds D A are used to. The carboxyl group is a functional group that contains a carbon-oxygen double bond and an OH group also attached to the same carbon atom.
30 Amino acids can be distinguished from one another by B A the number of R groups found on the amino acid moleculesB the chemical properties of their R groups. The group consists of a carbon C atom that. Another product commonly used that.
1 at an acidic pH amino groups in an R group tend to lonize by accepting a proton and have a positive charge2 at an acidic pH carboxyl groups in an R group are likely to be in an unionized state3 at an alkaline pH amino groups in an R group have a tendency to remain unionized and uncharged4 at an alkaline pH carboxyl groups in an R. One carbon atom one oxygen atom and one hydrogen atom. Another way to view it is as a carbonyl group CO that has a hydroxyl group O-H attached to the carbon atom.
A carboxyl group is one of many functional groups that attaches to larger molecules and gives them certain properties. Organic compounds that contain carboxyl groups are called carboxylic acids. What are the compounds in the Carboxyl group.
KnowledgeComprehension 40 In which of the structures are the atoms bonded by ionic bondsA A B B C CD C D and E only E none of the structures Answer. The following list includes several carboxyl group examples. Memorize flashcards and build a practice test to quiz yourself before your exam.
C the type of bond between the R group and the rest of the amino acid molecule. On the priority list of functional. So weve got heptanoic acid on our hands.
A carboxyl group also called a carboxy group is a characteristic group of atoms found in organic molecules. An organic compound consisting of a carboxyl group is termed as a carboxylic acid. Start studying the Chapter 1112 Functional Groups flashcards containing study terms like An organic compound that contains a hydroxyl group bonded to a carbon A hydrocarbon that contains one or more carbon-carbon double bonds An organic compound in which the carbon of a carbonyl.
They include acetic acid and amino acid. The carboxyl group COOH is present in all carboxylic acids. Write the IUPAC name of the following compound Answer.
The carboxyl group COOH is present in all carboxylic acids. The carboxyl group is made up of carbon atoms that have a hydrogen atom attached to them. Carboxylic acids are compound containing carboxyl structure.
Here are the structures of some common carboxylic acids. E none of the structures. One hydrogen atom and two carbon atoms d.
Secondly what is the purpose of a carboxyl group. A Carboxyl Group is a functional organic compound that comprises a double-bonded carbon atom linked to an oxygen group and a hydroxyl group through a single bond. An organic compound that binds to a carboxyl group is called a carboxylic acid.
Functional properties of the Carboxyl group Theres 2-acid can donate H because covalent bond between oxygen and hydrogen is so polar-found in cells in ionized form with charge 1-. Oxalic acid contains two COOH groups and citric acid contains three. The compound C H 3 C H 2 C O O H has three carbon atoms and is called propanoic acid.
Carboxylic compounds readily dissolve in neutral solvents to form weak. Carboxyl groups have the formula -C OOH usually written as -COOH or CO 2 H. Heptane is the parent chain which contains seven carbon atoms.
39 Which of the structures contain s a carboxyl functional group. One hydrogen atom two oxygen atoms and one carbon atom c. A A B B C CD C and E E none of the structures Answer.
One such acid is acetic acid found in vinegar. The carboxyl group is an important component of organic molecules such as amino acids fatty acids and acetic acids all of which play essential roles in biosynthesis and cellular respiration. Another way to view it is as a carbonyl group CO that has a hydroxyl group O-H attached to the carbon atom.
In chemistry the carboxyl group is an organic functional group consisting of a carbon atom thats double-bonded to an oxygen atom and singly bonded to a hydroxyl group. Carboxyl group is a functional organic compound. In the term carboxyl group group refers to a specific group of atoms COOH not to a group of similar types of compounds.
Carboxyl groups are commonly found in amino acids fatty acids and other biomolecules. In this structure of a carboxyl group a carbon atom is attached to an oxygen atom with the help of a double bond. One nitrogen atom and two hydrogen atoms b.
A carboxyl group also called a carboxy group is a characteristic group of atoms found in organic molecules. There are many members in this class of organic acids such as acetic acid. Oxygen and the hydroxyl group are bonded together.
The simplest carboxylic acid is the alkanoic acids. Oxalic acid contains two COOH groups and citric acid contains three. Carboxyl Group Definition.
A carboxyl group is made up of ____. A carboxylic acid is an organic compound that has a carboxyl group.
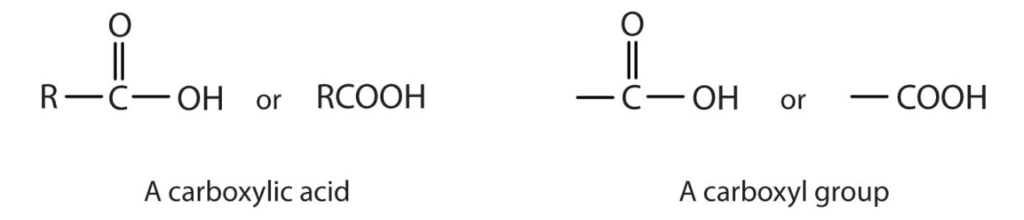
What Is A Carboxyl Group Education Is Around
Carboxyl Group Images Stock Photos Vectors Shutterstock
What Is A Molecule That Includes Hydroxyl Carboxyl And Amide Groups Quora

Comments
Post a Comment